First, blood is collected from the patient and is filtered by a machine in a process called apheresis.
In this process, white blood cells (including T cells) are separated from blood.

Investigating a new therapy to help
patients living with lupus nephritis.
Lupus Warrior
In the KYSA-1 clinical trial, doctors are looking at a type of treatment called CAR T-cell therapy in people with active lupus nephritis who do not get better with standard therapies.
Doctors want to find out how effective and safe CAR T-cell therapy is in people living with lupus nephritis.
Kyverna, along with the NIH, have developed the CAR T-cell therapy, KYV-101, for lupus nephritis. This is the treatment that will be used in the KYSA-1 clinical trial.
In the KYSA-1 clinical trial, participants’ T cells (a type of immune cell that helps to protect from diseases) will be collected, modified, and returned to the body so they can attack B cells (another type of immune cell) that, when unhealthy, can increase inflammation and the symptoms of lupus nephritis.

Clinical trials are research studies that look to find better ways to prevent, diagnose, or treat disease.
In a phase 1 clinical trial, doctors want to understand if a treatment is safe and well tolerated in humans.
Phase 1 trials are the first stage of human clinical trials and usually involve only a small number of people.
Phase 1 trials typically aim to find out:
In a phase 2 clinical trial, doctors want to understand if a treatment has a clinical benefit and is safe and well tolerated in humans.
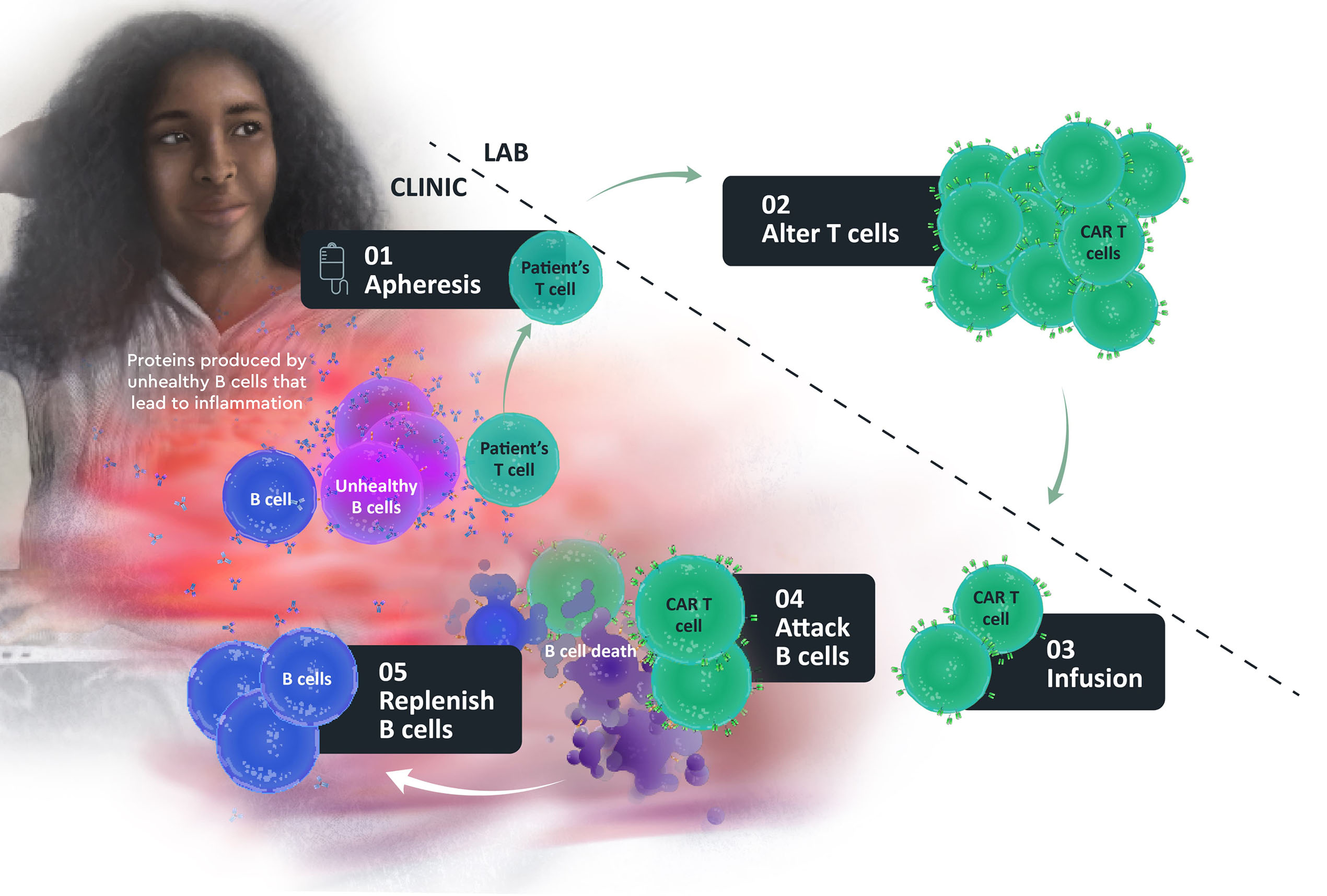
First, blood is collected from the patient and is filtered by a machine in a process called apheresis.
In this process, white blood cells (including T cells) are separated from blood.
T cells are altered in the laboratory so they can recognize, attack, and destroy the B cells.
Doctors then return the patient’s own altered T cells back to them through an infusion directly into their veins (intravenous (IV) infusion).
The CAR T cells attack B cells, including unhealthy B cells with the goal of helping to control, reduce, or reverse the disease.
The aim is for the body to replenish with healthy B cells.
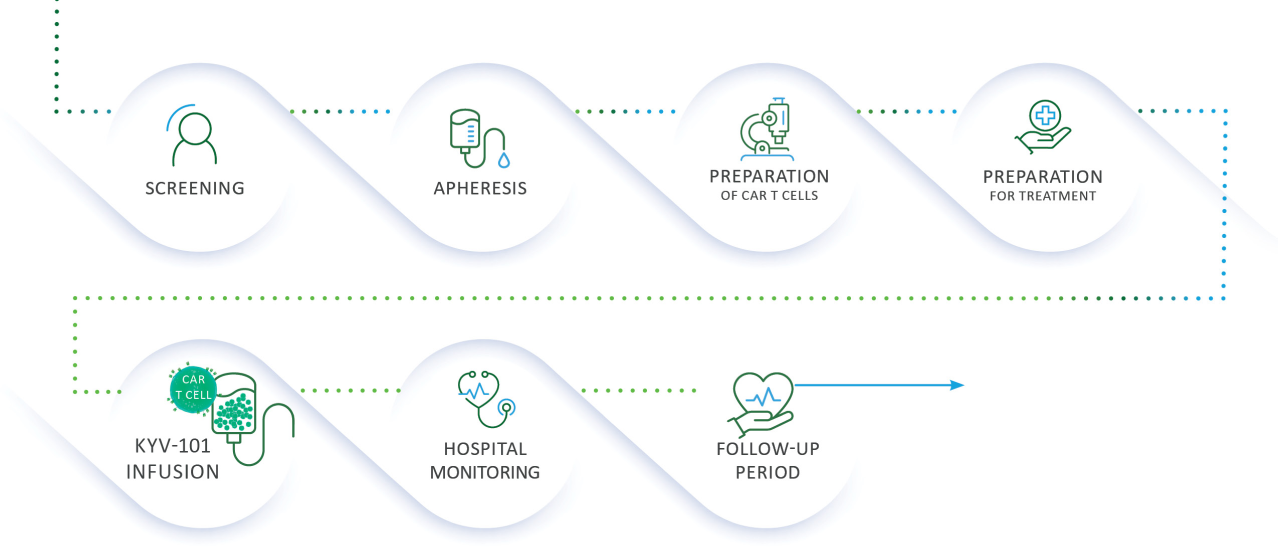
If you are selected to participate, one of the doctors running the trial (or someone on their behalf) will talk with you about what you can expect as a participant in the trial so that you understand and are comfortable with what it means to take part. This is called informed consent. If you are able to enroll in the trial, these are the key steps you will be involved in:
Serious and potentially life-threatening side effects can occur from CAR T-cell therapy including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) and typically resolve within the first month after treatment.
People receiving this treatment are always in the care of a doctor, who monitors them closely for these side effects and can treat their symptoms to help prevent their worsening.